ConsumerLab seals of approval for supplements with folate

Very recently ConsumerLab has published a new report title “B Vitamin Supplements and Energy Drinks (Including B Complexes, Niacin, B-6, B-12, Biotin, Thiamin, and Folate)” where several finished dietary supplements, commercialized in the US market and containing the (6S)-5-methyltetrahydrofolate (5-MTHF) – the biological active form of folate – or folic acid, have been tested.
With a long history of certifying supplements, ConsumerLab is the leading provider of independent test results and information to help consumers and healthcare professionals to identify the best quality health and nutrition products.
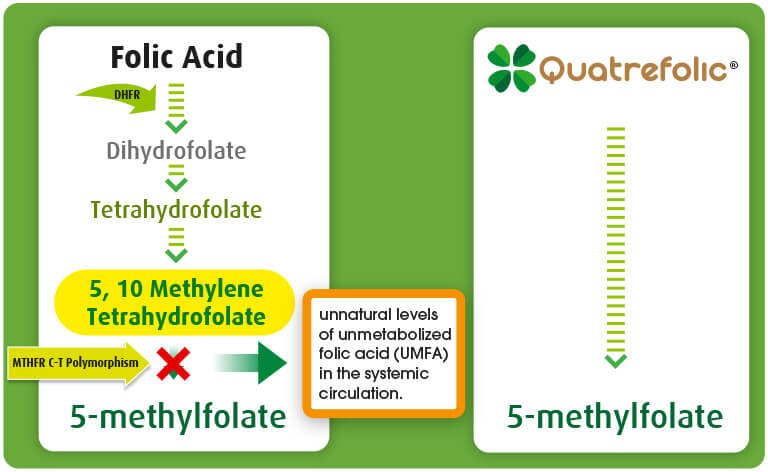
The report mentions the rational use of reduced folate and describes why they are suggested for people with a gene polymorphism of the enzyme MTHFR (methyltetrahydrofolate reductase) that drives an impairment in the metabolization of folic acid from food or supplements.
The report highlights the results for products containing one or more B vitamins. Supplements with vitamin B9 (folate) were stand-alone or in combination with other ingredients.
Among these, all those formulated with the biologically active reduced folate 5-MTHF contain Gnosis ingredients, and 66% of these counts the glucosamine salt Quatrefolic®. All supplements with Gnosis by Lesaffre’s ingredients passed the ConsumerLab tests.
Aberrant folate metabolism has adverse effects on human health, including increased risk for birth defects, cancer, cardiovascular disease, end-stage renal disease, diabetic neuropathy, and CNS disorders such as depression and cognitive impairment.
Among folate, Quatrefolic®, the Gnosis by Lesaffre’s proprietary innovActive folate made up of the glucosamine salt of (6S)-5-MTHF, is even more recognized as the folate of reference in the US market, as a direct source of the 5-MTHF that doesn’t need to be metabolized and can enter immediately folate-dependent pathways. It has established itself as the benchmark for the whole industry of folate derivatives.
The independent proof of the stability of Quatrefolic® in supplements, together with the already known advantages of this recommended reduced folate demonstrate again like it is a winning mix of efficacy, innovation, and safety.
Since the phase of development Gnosis has deliberately chosen to carry out a stringent stability test on Quatrefolic® performed according to ICH (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use) guidelines specifically required for pharmaceutical ingredients guaranteeing undependable and reliable criteria to claim the long-lasting chemical stability to its clients. Quatrefolic® shows a long-lasting chemical stability at both room and refrigerated conditions, guaranteeing purity and ensuring easy handling and storage.
Stability at room temperature might be a useful indicator to predict shelf life especially for combining the Quatrefolic® with other components such as vitamins and minerals.